Physisorption
Even though the interaction energy is very weak (~10–100 meV), physisorption plays an important role in nature.
For instance, the van der Waals attraction between surfaces and foot-hairs of geckos (see Synthetic setae) provides the remarkable ability to climb up vertical walls.
[4] Van der Waals forces originate from the interactions between induced, permanent or transient electric dipoles.
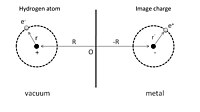
To give a simple illustration of physisorption, we can first consider an adsorbed hydrogen atom in front of a perfect conductor, as shown in Fig.
The adsorption process can be viewed as the interaction between this hydrogen atom and its image charges of both the nucleus and electron in the conductor.
By Taylor expansion in powers of |r| / |R|, this interaction energy can be further expressed as: One can find from the first non-vanishing term that the physisorption potential depends on the distance Z between adsorbed atom and surface as Z−3, in contrast with the r−6 dependence of the molecular van der Waals potential, where r is the distance between two dipoles.
The van der Waals binding energy can be analyzed by another simple physical picture: modeling the motion of an electron around its nucleus by a three-dimensional simple harmonic oscillator with a potential energy Va:[clarification needed] where me and ω are the mass and vibrational frequency of the electron, respectively.
As this atom approaches the surface of a metal and forms adsorption, this potential energy Va will be modified due to the image charges by additional potential terms which are quadratic in the displacements: Assuming the potential is well approximated as where If one assumes that the electron is in the ground state, then the van der Waals binding energy is essentially the change of the zero-point energy: This expression also shows the nature of the Z−3 dependence of the van der Waals interaction.
Also, by expressing the fourth-order correction in the Taylor expansion above as (aCvZ0) / (Z4), where a is some constant, we can define Z0 as the position of the dynamical image plane and obtain The origin of Z0 comes from the spilling of the electron wavefunction out of the surface.
As a result, the position of the image plane representing the reference for the space coordinate is different from the substrate surface itself and modified by Z0.
Table 1 shows the jellium model calculation for van der Waals constant Cv and dynamical image plane Z0 of rare gas atoms on various metal surfaces.
For the position of the dynamical image plane, it decreases with increasing dielectric function and is typically on the order of 0.2 Å.
This Pauli exclusion and repulsion are particularly strong for atoms with closed valence shells that dominate the surface interaction.
As a result, the minimum energy of physisorption must be found by the balance between the long-range van der Waals attraction and short-range Pauli repulsion.
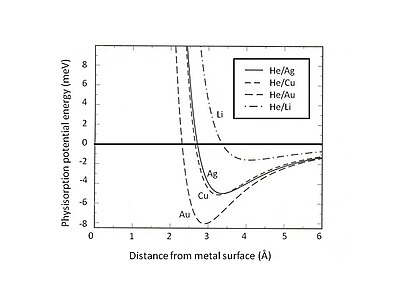
2 shows the physisorption potential energy of He adsorbed on Ag, Cu, and Au substrates which are described by the jellium model with different densities of smear-out background positive charges.
It can be found that the weak van der Waals interaction leads to shallow attractive energy wells (<10 meV).
These two are referred to as the chi hypothesis, the quantum mechanical derivation, and excess surface work, ESW.
where "ads" stands for "adsorbed", "m" stands for "monolayer equivalence" and "vap" is reference to the vapor pressure ("ads" and "vap" are the latest IUPAC convention but "m" has no IUAPC equivalent notation) of the liquid adsorptive at the same temperature as the solid sample.
Empirically, this plot was notice as being a very good fit to the isotherm by Polanyi[7][8][9] and also by deBoer and Zwikker[10] but not pursued.
A typical fit to good data on a homogeneous non-porous surface is shown in figure 3.