Phytochrome
They regulate the germination of seeds (photoblasty), the synthesis of chlorophyll, the elongation of seedlings, the size, shape and number and movement of leaves and the timing of flowering in adult plants.
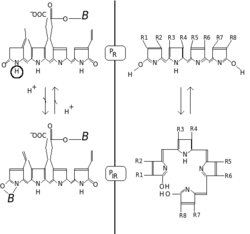
In the case of phytochrome the ground state is Pr, the r indicating that it absorbs red light particularly strongly.
The absorbance maximum is a sharp peak 650–670 nm, so concentrated phytochrome solutions look turquoise-blue to the human eye when viewed with white light.
But once a red photon has been absorbed, the pigment undergoes a rapid conformational change to form the Pfr state.
Here fr indicates that now not red but far-red (also called "near infra-red"; 705–740 nm) is differentially absorbed.
[7] Janoudi and his fellow coworkers wanted to see what type of phytochrome was responsible for causing phototropism to occur, and performed a series of experiments.
The experimenters utilized an apparatus that allowed for root apex to be zero degrees so that gravitropism could not be a competing factor.
The phytochrome chromophore is usually phytochromobilin, and is closely related to phycocyanobilin (the chromophore of the phycobiliproteins used by cyanobacteria and red algae to capture light for photosynthesis) and to the bile pigment bilirubin (whose structure is also affected by light exposure, a fact exploited in the phototherapy of jaundiced newborns).
In contrast to bilins, haem and chlorophyll carry a metal atom in the center of the ring, iron or magnesium, respectively.
[8] The Pfr state passes on a signal to other biological systems in the cell, such as the mechanisms responsible for gene expression.
[9] The phytochrome pigment was discovered by Sterling Hendricks and Harry Borthwick at the USDA-ARS Beltsville Agricultural Research Center in Maryland during a period from the late 1940s to the early 1960s.
Using a spectrograph built from borrowed and war-surplus parts, they discovered that red light was very effective for promoting germination or triggering flowering responses.
The phytochrome pigment was identified using a spectrophotometer in 1959 by biophysicist Warren Butler and biochemist Harold Siegelman.
In 1996 David Kehoe and Arthur Grossman at the Carnegie Institution at Stanford University identified the proteins, in the filamentous cyanobacterium Fremyella diplosiphon called RcaE with similarly to plant phytochrome that controlled a red-green photoreversible response called chromatic acclimation and identified a gene in the sequenced, published genome of the cyanobacterium Synechocystis with closer similarity to those of plant phytochrome.
Jon Hughes in Berlin and Clark Lagarias at UC Davis subsequently showed that this Synechocystis gene indeed encoded a bona fide phytochrome (named Cph1) in the sense that it is a red/far-red reversible chromoprotein.
Subsequently, phytochromes have been found in other prokaryotes including Deinococcus radiodurans and Agrobacterium tumefaciens.
In 2005, the Vierstra and Forest labs at the University of Wisconsin published a three-dimensional structure of a truncated Deinococcus phytochrome (PAS/GAF domains).
In 2008, two groups around Essen and Hughes in Germany and Yang and Moffat in the US published the three-dimensional structures of the entire photosensory domain.
In 2014 it was confirmed by Takala et al that the refolding occurs even for the same phytochrome (from Deinococcus) as a function of illumination conditions.
In all cases the resulting plants had conspicuously short stems and dark green leaves.
Harry Smith and co-workers at Leicester University in England showed that by increasing the expression level of phytochrome A (which responds to far-red light), shade avoidance responses can be altered.
In 2002, the light-induced interaction between a plant phytochrome and phytochrome-interacting factor (PIF) was used to control gene transcription in yeast.