Isotopes of plutonium
Plutonium (94Pu) is an artificial element, except for trace quantities resulting from neutron capture by uranium, and thus a standard atomic weight cannot be given.
The even-mass isotopes are fertile but not fissile and also have a lower probability (cross section) of neutron capture; therefore, they tend to accumulate in nuclear fuel used in a thermal reactor, the design of nearly all nuclear power plants today.
241Pu has a half-life of 14 years, and has slightly higher thermal neutron cross sections than 239Pu for both fission and absorption.
Because 243Pu has little opportunity to capture an additional neutron before decay, the nuclear fuel cycle does not produce the long-lived 244Pu in significant quantity.
However, isotopic separation would be quite expensive compared to another method: when 235U captures a neutron, it is converted to an excited state of 236U.
Some of the excited 236U nuclei undergo fission, but some decay to the ground state of 236U by emitting gamma radiation.
After chemical separation of neptunium, 237Np is again irradiated by reactor neutrons to be converted to 238Np, which decays to 238Pu with a half-life of 2 days.
[1] The presence of 240Pu limits the plutonium's use in a nuclear bomb, because a neutron from spontaneous fission starts the chain reaction prematurely, causing an early release of energy that disperses the core before full implosion is reached.
However, modern nuclear weapons use fusion boosting, which mitigates the predetonation problem; if the pit can generate a nuclear weapon yield of even a fraction of a kiloton, which is enough to start deuterium–tritium fusion, the resulting burst of neutrons will fission enough plutonium to ensure a yield of tens of kilotons.
Theoretically, pure 239Pu could be used in a gun-type bomb, but achieving this level of purity is prohibitively difficult.
While it created delays and headaches during the Manhattan Project because of the need to develop implosion technology, those same difficulties are a barrier to nuclear proliferation.

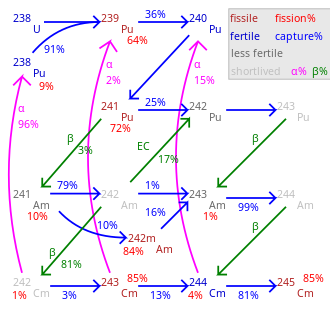
Transmutation speed not shown and varies greatly by nuclide. 245 Cm– 248 Cm are long-lived with negligible decay.
