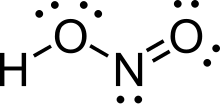
Nitrous acid
In the gas phase, the planar nitrous acid molecule can adopt both a syn and an anti form.
It can be seen that the values of Eocell for these reactions are similar, but nitric acid is a more powerful oxidizing agent.
Such salts are widely used in organic synthesis, e.g., for the Sandmeyer reaction and in the preparation azo dyes, brightly colored compounds that are the basis of a qualitative test for anilines.
[9] Nitrous acid is used to destroy toxic and potentially explosive sodium azide.
When this reaction takes place on the surface of atmospheric aerosols, the product readily photolyses to hydroxyl radicals.