Polymer chemistry
Many materials have polymeric structures, from fully inorganic metals and ceramics to DNA and other biological molecules.
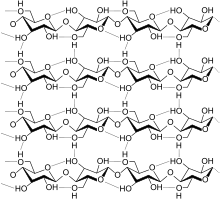
In 1884 Hilaire de Chardonnet started the first artificial fiber plant based on regenerated cellulose, or viscose rayon, as a substitute for silk, but it was very flammable.
[5] In 1907 Leo Baekeland invented the first polymer made independent of the products of organisms, a thermosetting phenol-formaldehyde resin called Bakelite.
Around the same time, Hermann Leuchs reported the synthesis of amino acid N-carboxyanhydrides and their high molecular weight products upon reaction with nucleophiles, but stopped short of referring to these as polymers, possibly due to the strong views espoused by Emil Fischer, his direct supervisor, denying the possibility of any covalent molecule exceeding 6,000 daltons.
[7] The chemist Hermann Staudinger first proposed that polymers consisted of long chains of atoms held together by covalent bonds, which he called macromolecules.
Before Staudinger, polymers were thought to be clusters of small molecules (colloids), without definite molecular weights, held together by an unknown force.
Paul Flory was awarded the Nobel Prize in Chemistry in 1974 for his work on polymer random coil configurations in solution in the 1950s.
Karl Ziegler and Giulio Natta received a Nobel Prize for their discovery of catalysts for the polymerization of alkenes.
Illustrative of the quantitative aspects of polymer chemistry, particular attention is paid to the number-average and weight-average molecular weights
The polynucleic acids DNA and RNA are derived from phosphorylated sugars with pendant nucleotides that carry genetic information.
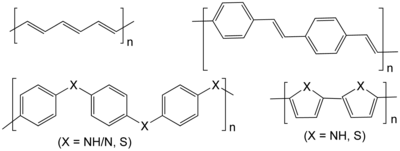
Thermoplastic polymers include polyethylene, teflon, polystyrene, polypropylene, polyester, polyurethane, Poly(methyl methacrylate), polyvinyl chloride, nylons, and rayon.