Polythiophene
The optical properties of these materials respond to environmental stimuli, with dramatic color shifts in response to changes in solvent, temperature, applied potential, and binding to other molecules.
An idealized stoichiometry is shown using the oxidant [A]PF6: In principle, PT can be n-doped using reducing agents, but this approach is rarely practiced.
Iodine and bromine produce highly conductive materials,[9] which are unstable owing to slow evaporation of the halogen.
As an approximation, the conjugated backbone can be considered as a real-world example of the "electron-in-a-box" solution to the Schrödinger equation; however, the development of refined models to accurately predict absorption and fluorescence spectra of well-defined oligo(thiophene) systems is ongoing.
[20] The effective conjugation length of polythiophene derivatives depend on the chemical structure of side chains,[21] and thiophene backbones.
This is attributed to formation of a compact coil structure, which can form hydrogen bonds with PVA upon partial deprotonation of the acetic acid group.
PTs exhibit absorption shifts due to application of electric potentials (electrochromism),[25] or to introduction of alkali ions (ionochromism).
"[29] Nonetheless, intense interest has focused on soluble polythiophenes, which usually translates to polymers derived from 3-alkylthiophenes, which give the so-called polyalkylthiophenes (PATs).
[33] PATs prepared using Rieke zinc formed "crystalline, flexible, and bronze-colored films with a metallic luster".
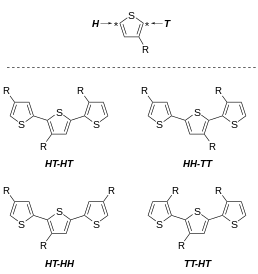
The difference between absorption and emission maxima, the Stokes shift, also increases with HH dyad content, which they attributed to greater relief from conformational strain in the first excited state.
[35] Water-soluble PT's are represented by sodium poly(3-thiophenealkanesulfonate)s.[36] In addition to conferring water solubility, the pendant sulfonate groups act as counterions, producing self-doped conducting polymers.
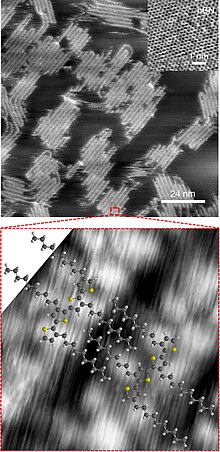
Atomic force microscopy (AFM) images showed a significant increase in long-range order after heating.
[46] In an electrochemical polymerization, a solution containing thiophene and an electrolyte produces a conductive PT film on the anode.
The stoichiometry of the electropolymerization is: The degree of polymerization and quality of the resulting polymer depends upon the electrode material, current density, temperature, solvent, electrolyte, presence of water, and monomer concentration.
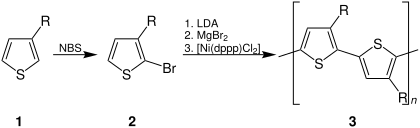
[53][54] In contrast to methods that require brominated monomers, the oxidative polymerization of thiophenes using ferric chloride proceeds at room temperature.
This method has proven to be extremely popular; antistatic coatings are prepared on a commercial scale using ferric chloride.
[29] Slow addition of ferric chloride to the monomer solution produced poly(3-(4-octylphenyl)thiophene)s with approximately 94% H–T content.
[29] Exhaustive Soxhlet extraction after polymerization with polar solvents was found to effectively fractionate the polymer and remove residual catalyst before NMR spectroscopy.
[30] Using a lower ratio of catalyst to monomer (2:1, rather than 4:1) may increase the regioregularity of poly(3-dodecylthiophene)s.[58] Andreani et al. reported higher yields of soluble poly(dialkylterthiophene)s in carbon tetrachloride rather than chloroform, which they attributed to the stability of the radical species in carbon tetrachloride.
[59] Higher-quality catalyst, added at a slower rate and at reduced temperature, was shown to produce high molecular weight PATs with no insoluble polymer residue.
Niemi et al. reported that polymerization was only observed in solvents where the catalyst was either partially or completely insoluble (chloroform, toluene, carbon tetrachloride, pentane, and hexane, and not diethyl ether, xylene, acetone, or formic acid), and speculated that the polymerization may occur at the surface of solid ferric chloride.
[63] Barbarella et al. studied the oligomerization of 3-(alkylsulfanyl)thiophenes, and concluded from their quantum mechanical calculations, and considerations of the enhanced stability of the radical cation when delocalized over a planar conjugated oligomer, that a radical cation mechanism analogous to that generally accepted for electrochemical polymerization was more likely.
As an example of a static application, poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonate) (PEDOT-PSS) product ("Clevios P") from Heraeus has been extensively used as an antistatic coating (as packaging materials for electronic components, for example).
The thin layer of PEDOT:PSS is virtually transparent and colorless, prevents electrostatic discharges during film rewinding, and reduces dust buildup on the negatives after processing.
PEDOT-coated windows and mirrors become opaque or reflective upon the application of an electric potential, a manifestation of its electrochromic properties.
In addition to biosensor applications, PTs can also be functionalized with receptors for detecting metal ions or chiral molecules as well.