Polymer degradation
Polymers and particularly plastics are subject to degradation at all stages of their product life cycle, including during their initial processing, use, disposal into the environment and recycling.
[1] The rate of this degradation varies significantly; biodegradation can take decades, whereas some industrial processes can completely decompose a polymer in hours.
For instance, polymer stabilizers ensure plastic items are produced with the desired properties, extend their useful lifespans, and facilitate their recycling.
The major chemical changes are oxidation and chain scission, leading to a reduction in the molecular weight and degree of polymerization of the polymer.
Plastics exist in huge variety, however several types of commodity polymer dominate global production: polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polyethylene terephthalate (PET, PETE), polystyrene (PS), polycarbonate (PC), and poly(methyl methacrylate) (PMMA).
The majority (PP, PE, PVC, PS and PMMA) are addition polymers with all-carbon backbones that are more resistant to most types of degradation.
[2] Polymers will oxidise under these conditions, but even in the absence of air, these temperatures are sufficient to cause thermal degradation in some materials.
Pelletised material prepared in this may also be pre-dried in an oven to remove trace moisture prior to its final melting and moulding into plastic items.
[3] Although oxygen levels inside processing equipment are usually low, it cannot be fully excluded and thermal-oxidation will usually take place more readily than degradation that is exclusively thermal (i.e. without air).
Some plastic items, however, can experience long service-lives in aggressive environments, particularly those where they are subject to prolonged heat or chemical attack.
Many electric items like transformers, microprocessors or high-voltage cables operate at elevated temperatures for years, or even decades, resulting in low-level but continuous thermal oxidation.
This can be exacerbated by direct contact with metals, which can promote the formation of free-radicals, for instance, by the action of Fenton reactions on hydroperoxides.
[14][15] Polymer degradation by galvanic action was first described in the technical literature in 1990 by Michael C. Faudree, an employee at General Dynamics, Fort Worth Division.
[16][17] The phenomenon has been referred to as the "Faudree Effect",[18] and can possibly be used as a sustainable process to degrade non-recyclable thermoset plastics, and also has had implications for preventing corrosion on aircraft for safety such as changes in design.
[19][20] When carbon-fiber-reinforced polymer is attached to a metal surface, the carbon fiber can act as a cathode if exposed to water or sufficient humidity, resulting in galvanic corrosion.
These act as photoinitiators to give complex free radical chain reactions where the mechanisms of autoxidation and photodegradation combine.
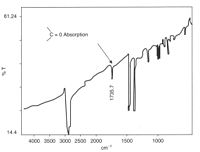
Condensation polymers like polyesters,[31] polyamides, polyurethanes and polycarbonates can be degraded by hydrolysis of their carbonyl groups, to give lower molecular weight molecules.
Such reactions are exceedingly slow at ambient temperatures, however, they remain a significant source of degradation for these materials, particularly in the marine environment.
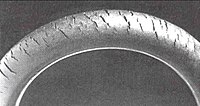
Ozone cracks in products under tension are always oriented at right angles to the strain axis, so will form around the circumference in a rubber tube bent over.
The major appeal of biodegradation is that, in theory, the polymer will be completely consumed in the environment without needing complex waste management and that the products of this will be non-toxic.
Long-chain polymers with all-carbon backbones like polyolefins, polystyrene and PVC will not degrade by biological action alone[34] and must first be oxidised to create chemical groups which the enzymes can attack.
[37] The act of recycling plastic degrades its polymer chains, usually as a result of thermal damage similar to that seen during initial processing.
Technologies developed to enhance the biodegradation of plastic can also conflict with its recycling, with oxo-biodegradable additives, consisting of metallic salts of iron, magnesium, nickel, and cobalt, increasing the rate of thermal degradation.
Condensation polymers baring cleavable groups such as esters and amides can also be completely depolymerised by hydrolysis or solvolysis.
[51][52] Hindered amine light stabilizers (HALS) stabilise against weathering by scavenging free radicals that are produced by photo-oxidation of the polymer matrix.


PP: polypropylene , PE: polyethylene , PVC: Polyvinyl chloride , PS: Polystyrene , PET: Polyethylene terephthalate