Polymerization
In chemical compounds, polymerization can occur via a variety of reaction mechanisms that vary in complexity due to the functional groups present in the reactants[3] and their inherent steric effects.
[3] As alkenes can polymerize in somewhat straightforward radical reactions, they form useful compounds such as polyethylene and polyvinyl chloride (PVC),[3] which are produced in high tonnages each year[3] due to their usefulness in manufacturing processes of commercial products, such as piping, insulation and packaging.
Other monomer units, such as formaldehyde hydrates or simple aldehydes, are able to polymerize themselves at quite low temperatures (ca.
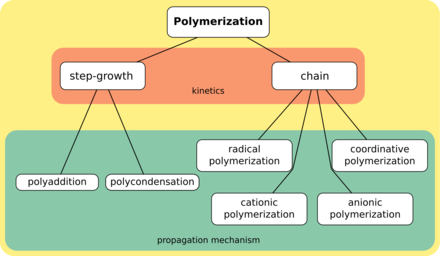
[6][7] Step-growth polymers are formed by independent reaction steps between functional groups of monomer units, usually containing heteroatoms such as nitrogen or oxygen.
For example, polyester chains grow by reaction of alcohol and carboxylic acid groups to form ester links with loss of water.
Chain-growth polymerization is involved in the manufacture of polymers such as polyethylene, polypropylene, polyvinyl chloride (PVC), and acrylate.
Diverse methods are employed to manipulate the initiation, propagation, and termination rates during chain polymerization.
A related issue is temperature control, also called heat management, during these reactions, which are often highly exothermic.
Although the polymer dispersity and molecular weight may be improved, these methods may introduce additional processing requirements to isolate the product from a solvent.
[11] Photopolymerization can be used as a photographic or printing process because polymerization only occurs in regions which have been exposed to light.