Photopolymer
A photopolymer or light-activated resin is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum.
[1] These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light.
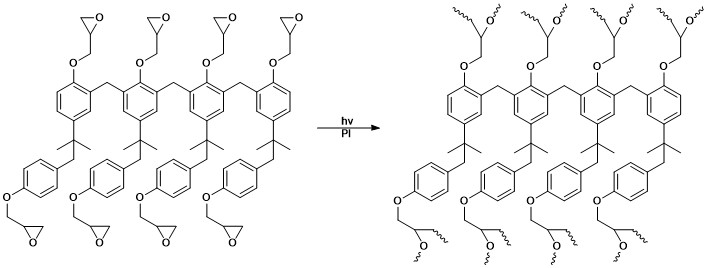
An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing.
Changes in structural and chemical properties can be induced internally by chromophores that the polymer subunit already possesses, or externally by addition of photosensitive molecules.
Photopolymers undergo a process called curing, where oligomers are cross-linked upon exposure to light, forming what is known as a network polymer.
Photoinitiators are compounds that upon radiation of light decompose into reactive species that activate polymerization of specific functional groups on the oligomers.
However, the development of dye-based photoinitiator systems have allowed for the use of visible light, having the potential advantages of being simpler and safer to handle.
[1][4] The general process involves doping a batch of neat polymer with small amounts of photoinitiator, followed by selective radiation of light, resulting in a highly cross-linked product.
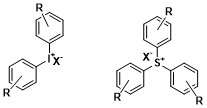
In order to satisfy this, liquid neat oligomer can be doped with either anionic or cationic photoinitiators that will initiate polymerization only when radiated with light.
[1] One of the advantages to using cationic photopolymerization is that once the polymerization has begun it is no longer sensitive to oxygen and does not require an inert atmosphere to perform well.
Onium salts generally absorb short wavelength light in the UV region spanning from 225–300 nm.
The first to demonstrate the photoinduced free radical chain reaction of vinyl bromide was Ivan Ostromislensky, a Russian chemist who also studied the polymerization of synthetic rubber.
[1] In the free radical mechanism of radiation curable systems, light absorbed by a photoinitiator generates free-radicals which induce cross-linking reactions of a mixture of functionalized oligomers and monomers to generate the cured film [13] Photocurable materials that form through the free-radical mechanism undergo chain-growth polymerization, which includes three basic steps: initiation, chain propagation, and chain termination.
In photocurable materials the propagation step involves reactions of the chain radicals with reactive double bonds of the prepolymers or oligomers.
Most composites that cure through radical chain growth contain a diverse mixture of oligomers and monomers with functionality that can range from 2-8 and molecular weights from 500 to 3000.
[5] Typically these oligomers and monomers alone do not absorb sufficient energy for the commercial light sources used, therefore photoinitiators are included.
[13] Benzophenone, xanthones, and quinones are examples of abstraction type photoinitiators, with common donor compounds being aliphatic amines.
Oligomers are typically epoxides, urethanes, polyethers, or polyesters, each of which provide specific properties to the resulting material.
Acrylated urethane oligomers are typically abrasion resistant, tough, and flexible, making ideal coatings for floors, paper, printing plates, and packaging materials.
[4] The monomers used in radiation curable systems help control the speed of cure, crosslink density, final surface properties of the film, and viscosity of the resin.
Dentistry is one field in which free radical photopolymers have found wide usage as adhesives, sealant composites, and protective coatings.
These dental composites are based on a camphorquinone photoinitiator and a matrix containing methacrylate oligomers with inorganic fillers such as silicon dioxide.
[14] Conventional halogen bulbs, argon lasers and xenon arc lights are currently used in clinical practice.
[15] Photocurable adhesives are also used in the production of catheters, hearing aids, surgical masks, medical filters, and blood analysis sensors.
Although photopolymers show promise for a wide range of new biomedical applications, biocompatibility with photopolymeric materials must still be addressed and developed.
Photopolymers used in 3D imaging processes require sufficient cross-linking and should ideally be designed to have minimal volume shrinkage upon polymerization in order to avoid distortion of the solid object.
Common monomers utilized for 3D imaging include multifunctional acrylates and methacrylates, often combined with a non-polymeric component in order to reduce volume shrinkage.
[12] A competing composite mixture of epoxide resins with cationic photoinitiators is becoming increasingly used since their volume shrinkage upon ring-opening polymerization is significantly below those of acrylates and methacrylates.
[25] Light-activated resins have found a place in floor refinishing applications, offering an instant return to service not available with any other chemical due to the need to cure at ambient temperatures.
Because of application constraints, these coatings are exclusively UV cured with portable equipment containing high intensity discharge lamps.