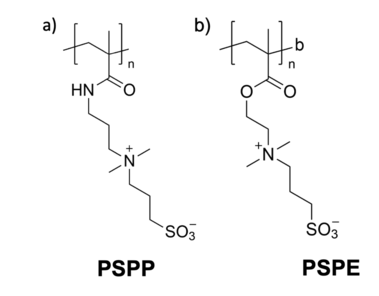
Polysulfobetaine
These properties are mainly referred to a tightly bound hydration layer around each zwitterionic group, which effectively suppresses protein adsorption and thus, improves anti-fouling behavior.
[6][11] However, the synthesis of polysulfobetaines is often limited by their poor solubility in most solvents and at present, only few sulfobetaine monomers that are suited for free radical polymerization, are commercially available.
Due to the neutralization of the charges, repulsive and attractive interactions are present between the individual polymer chains and inner salt are formed.
The temperature of this phase transition, often called clearing point, is very sensitive to molar mass, polymer architecture, solvent isotopes, e.g., H2O/D2O, and especially to the addition of salts to the solution.
[23][24][25] Thin films made from polysulfobetaines also feature a thermo-responsiveness, however, the phase transition is strongly shifted, which is mainly addressed to the increased polymer concentration and the altered polymer-polymer and polymer-water interactions.