Temperature-responsive polymer
In a stricter sense, thermoresponsive polymers display a miscibility gap in their temperature-composition diagram.
Promising areas of application are tissue engineering,[2] liquid chromatography,[3][4] drug delivery[2][5] and bioseparation.
[6] Only a few commercial applications exist, for example, cell culture plates coated with an LCST-polymer.The theory of thermoresponsive polymer (similarly, microgels) begins in the 1940s with work from Flory and Huggins who both independently produced similar theoretical expectations for polymer in solution with varying temperature.
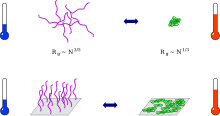
Thermoresponsive polymer chains in solution adopt an expanded coil conformation.
When mechanisms which reduce surface tension are absent, the globules aggregate, subsequently causing turbidity and the formation of visible particles.
In strictly binary mixtures the composition of the coexisting phases can be determined by drawing tie-lines.
It is convenient to add the glass transition curve to the phase diagram, although it is no real equilibrium.
A model for the phenomenological description of polymer phase diagrams was developed by Flory and Huggins (see Flory–Huggins solution theory).
Whether a polymer shows LCST and/or UCST behavior can be derived from the temperature-dependence of the interaction parameter (see figure).
For these reasons, classical Flory-Huggins theory cannot provide much insight into the molecular origin of miscibility gaps.
The described behavior can be exploited in tissue engineering since the adhesion of cells is strongly dependent on the hydrophilicity/hydrophobicity.
[16][17] Thermoresponsive polymer gels show a discontinuous change of the degree of swelling with temperature.
At the volume phase transition temperature (VPTT) the degree of swelling changes drastically.
[18] More sophisticated "catch and release" techniques have been elaborated in combination with lithography[19] and molecular imprinting.
[29] In baking, thermoreversible glazes such as pectin are prized for their ability to set and then reset after melting,[30] and are used in nappage and other processes to ensure a smooth final surface for a presented dish.
[11] The cloud point can be affected by many structural parameters of the polymer like the hydrophobic content,[25][26][27][28][34] architecture[25][26] and even the molar mass.
[27][35] The cloud points upon cooling and heating of a thermoresponsive polymer solution do not coincide because the process of equilibration takes time.
[37] Also desirable for many applications is a sharp phase transition, which is reflected by a sudden drop in transmittance.
[36] As a result of phase separation, thermoresponsive polymer systems can form well-defined self-assembled nanostructures with a number of different practical application such as in drug and gene delivery, tissue engineering, etc.
In order to establish the required properties for applications, a rigorous characterization of the phase separation phenomenon can be carried out by different spectroscopic and calorimetric methods, including nuclear magnetic resonance (NMR) , dynamic light scattering (DLS), small-angle X-ray scattering (SAXS), infrared spectroscopy (IR), Raman spectroscopy, and Differential scanning calorimetry (DSC).
[38] Due to the low entropy of mixing, miscibility gaps are often observed for polymer solutions.
[39] Examples for organic polymer solutions with UCST are polystyrene in cyclohexane,[37][40] polyethylene in diphenylether[41][42] or polymethylmethacrylate in acetonitrile.
[59][60][61][62] By contrast, polyacrylic acid displays UCST behavior solely at high ionic strength.
[63] Examples for polymer that show UCST behavior in pure water as well as under physiological conditions are poly(N-acryloylglycinamide),[64][65][66][67] ureido-functionalized polymers,[68] copolymers from N-vinylimidazole and 1-vinyl-2-(hydroxylmethyl)imidazole[69] or copolymers from acrylamide and acrylonitrile.
Small amounts of ionic groups may suppress phase separation in pure water.
[74][75][76] The order of the individual phase transitions depends on the relative positions of the UCST and LCST.
Thus, upon temperature change the roles of the soluble and insoluble polymer blocks are reversed and this structural inversion is typically called ‘schizophrenic’ in the literature.
[77][78][79] Besides the fundamental interest in the mechanism of this behavior, such block copolymers have been proposed for application in smart emulsification, drug delivery, and rheology control.
[80][81][82] Schizophrenic diblock copolymer have also been applied as thin films for potential use as sensors, smart coatings or nanoswitches, and soft robotics.