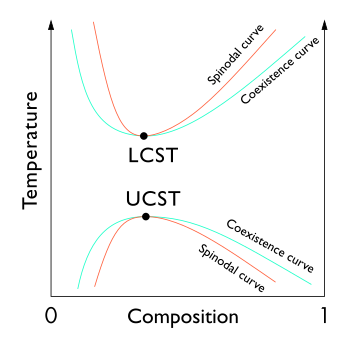Upper critical solution temperature
For example, hexane-nitrobenzene mixtures have a UCST of 19 °C (66 °F), so that these two substances are miscible in all proportions above 19 °C (66 °F) but not at lower temperatures.
[2]: 186 In the phase diagram of the mixture components, the UCST is the shared maximum of the concave down spinodal and binodal (or coexistence) curves.
The phase separation at the UCST is in general driven by unfavorable energetics; in particular, interactions between components favor a partially demixed state.
As shown in the diagram, for polymer solutions the LCST is higher than the UCST, so that there is a temperature interval of complete miscibility, with partial miscibility at both higher and lower temperatures.
[6] The seminal statistical mechanical model for the UCST of polymers is the Flory–Huggins solution theory.