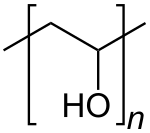
Polyvinyl alcohol
It has the idealized formula [CH2CH(OH)]n. It is used in papermaking, textile warp sizing, as a thickener and emulsion stabilizer in polyvinyl acetate (PVAc) adhesive formulations, in a variety of coatings, and 3D printing.
[3][4] Without an externally added crosslinking agent, PVA solution can be gelled through repeated freezing-thawing, yielding highly strong, ultrapure, biocompatible hydrogels which have been used for a variety of applications such as vascular stents, cartilages, contact lenses, etc.
Specific uses include cartilage replacements, contact lenses, laundry detergent pods and eye drops.
[9] Medically, PVA-based microparticles have received FDA 510(k) approval to be used as embolisation particles to be used for peripheral hypervascular tumors.
[11] In biomedical engineering research, PVA has also been studied for cartilage, orthopaedic applications,[12] and potential materials for vascular graft.
[citation needed] PVA may be used as an adhesive during preparation of stool samples for microscopic examination in pathology.
In terms of microstructure, it is composed mainly of 1,3-diol linkages [−CH2−CH(OH)−CH2−CH(OH)−], but a few percent of 1,2-diols [−CH2−CH(OH)−CH(OH)−CH2−] occur, depending on the conditions for the polymerization of the vinyl ester precursor.
Tests showed that fish (guppies) are not harmed, even at a poly(vinyl alcohol) concentration of 500 mg/L of water.