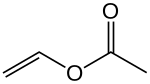
Vinyl acetate
The major industrial route involves the reaction of ethylene and acetic acid with oxygen in the presence of a palladium catalyst.
Beta-hydride elimination would generate vinyl acetate and a palladium hydride, which would be oxidized to give hydroxide.
However, RAFT (or more specifically, MADIX) polymerization offers a convenient method of controlling the synthesis of PVA by the addition of a xanthate or a dithiocarbamate chain transfer agent.
[3] On January 31, 2009, the Government of Canada's final assessment concluded that exposure to vinyl acetate is not harmful to human health.
In the context of large-scale release into the environment, it is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S.
11002), under which it "does not meet toxicity criteria[,] but because of its acute lethality, high production volume [or] known risk is considered a chemical of concern".
By this law, it is subject to strict reporting requirements by facilities that produce, store, or use it in quantities greater than 1000 pounds.