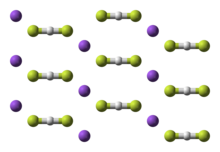
Potassium bifluoride
Potassium bifluoride is the inorganic compound with the formula K[HF2].
This colourless salt consists of the potassium cation (K+) and the bifluoride anion ([HF2]−).
Sodium bifluoride is related and is also of commercial use as an etchant as well as in cleaning products.
[3] The salt was prepared by Edmond Frémy by treating potassium carbonate or potassium hydroxide with hydrofluoric acid: With one more equivalent of HF, K[H2F3] (CAS RN 12178-06-2, m.p.
71.7 °C[4]) is produced: Thermal decomposition of K[HF2] gives hydrogen fluoride: The industrial production of fluorine entails the electrolysis of molten K[HF2] and K[H2F3].