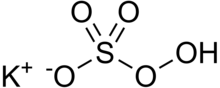
Potassium peroxymonosulfate
Potassium peroxymonosulfate is widely used as an oxidizing agent, for example, in pools and spas (usually referred to as monopersulfate or "MPS").
[4] Oxone, which is commercially available, is produced from peroxysulfuric acid, which is generated in situ by combining oleum and hydrogen peroxide.
It whitens dentures,[5] oxidizes organic contaminants in swimming pools,[6]and cleans chips for the manufacture of microelectronics.
[15] Ammonium, sodium, and potassium salts of H are used in the plastics industry as radical initiators for polymerization.
They are also used as etchants, oxidative desizing agents for textile fabrics, and for decolorizing and deodorizing oils.