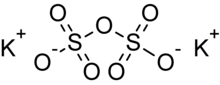
Potassium pyrosulfate
The geometry can be visualized as a tetrahedron with two corners sharing the SO4 anion's configuration and a centrally bridged oxygen atom.
[4] A semi-structural formula for the pyrosulfate anion is O3SOSO32−.
The oxidation state of sulfur in this compound is +6.
Potassium pyrosulfate is used in analytical chemistry; samples are fused with potassium pyrosulfate, (or a mixture of potassium pyrosulfate and potassium fluoride) to ensure complete dissolution prior to a quantitative analysis.
[5][6] The compound is also present in a catalyst in conjunction with vanadium(V) oxide in the industrial production of sulfur trioxide.