Potassium sulfide
Most commonly, the term potassium sulfide refers loosely to this mixture, not the anhydrous solid.
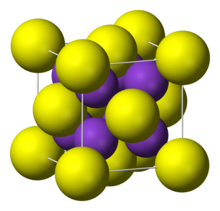
It adopts "antifluorite structure," which means that the small K+ ions occupy the tetrahedral (F−) sites in fluorite, and the larger S2− centers occupy the eight-coordinate sites.
[3] It can be produced by heating K2SO4 with carbon (coke): In the laboratory, pure K2S may be prepared by the reaction of potassium and sulfur in anhydrous ammonia.
[4] Sulfide is highly basic, consequently K2S completely and irreversibly hydrolyzes in water according to the following equation: For many purposes, this reaction is inconsequential since the mixture of SH− and OH− behaves as a source of S2−.
[3] Potassium sulfides are formed when black powder is burned and are important intermediates in many pyrotechnic effects, such as senko hanabi and some glitter formulations.