Potassium tert-butoxide
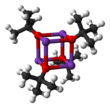
Mild Lewis basic solvents such as THF and diethyl ether do not break up the tetrameric structure, which persists in the solid, in solution and even in the gas phase.
Its steric bulk inhibits the group from participating in nucleophilic addition, such as in a Williamson ether synthesis or related SN2 reactions.
[citation needed] Substrates that are deprotonated by potassium t-butoxide include terminal acetylenes and active methylene compounds.
[5][6] Potassium tert-butoxide can abstract a beta-proton from alkylammonium cations, leading to the Hofmann product via an elimination reaction.
Potassium tert-butoxide catalyzes the reaction of hydrosilanes and heterocyclic compounds to give the silyl derivatives, with release of H2.