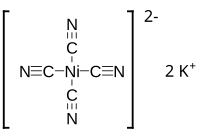
Potassium tetracyanonickelate
The salt consists of potassium ions and the tetracyanonickelate coordination complex, which is square planar.
[1] This columnar structure resembles those of the other [M(CN)4]2- anions of the heavy congeners of the group 10 metals (M = Pd, Pt).
The synthesis is often conducted stepwise, beginning with precipitating solid nickel dicyanide coordination polymer.
This route allows removal of excess potassium salts:[2] This procedure yields the monohydrate.
The complex binds four equivalents of boron trifluoride: Cyanide is a sufficient pi-acceptor ligand to allow reduction of K2Ni(CN)4 to the Ni(0) derivative.