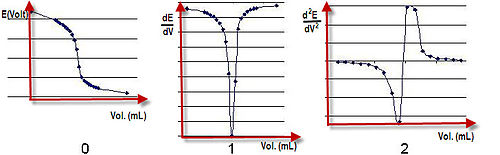
Potentiometric titration
No indicator is used; instead the electric potential is measured across the analyte, typically an electrolyte solution.
The indicator electrode forms an electrochemical half-cell with the interested ions in the test solution.
A graph of potential against volume added can be drawn and the end point of the reaction is halfway between the jump in voltage.
The first potentiometric titration was carried out in 1893 by Robert Behrend at Ostwald's Institute in Leipzig.
This decreased the osmotic pressure of mercury (I) ions on the side and creates a potential difference.
[1] Wilhelm Böttger then developed the tool of potentiometric titration while working at Ostwald's Institute.