Preferred Reporting Items for Systematic Reviews and Meta-Analyses
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) is an evidence-based minimum set of items aimed at helping scientific authors to report a wide array of systematic reviews and meta-analyses, primarily used to assess the benefits and harms of a health care intervention.
[citation needed] In 1987, Cynthia Mulrow examined for the first time the methodological quality of a sample of 50 review articles published in four leading medical journals between 1985 and 1986.
She found that none met a set of eight explicit scientific criteria, and that the lack of quality assessment of primary studies was a major pitfall in these reviews.
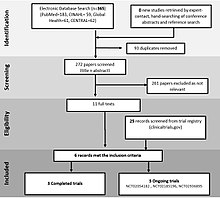
The rationale behind the inclusion of such a diagram is to increase the transparency of decisions made by the researcher for including or excluding certain studies, which may subsequently introduce biases in the overall measure of effect.
[citation needed] Recent surveys of leading medical journals evaluated the extent to which the PRISMA Statement has been incorporated into their Instructions to Authors.
[citation needed] Approximately 174 journals in the health sciences endorse the PRISMA Statement for the reporting of systematic reviews and meta-analysis published in their collections.