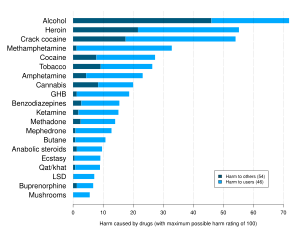
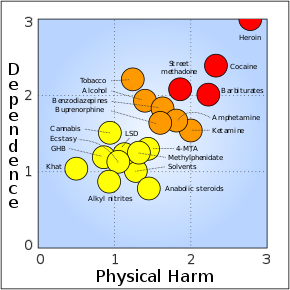
Buprenorphine
[21] Side effects may include respiratory depression (decreased breathing), sleepiness, adrenal insufficiency, QT prolongation, low blood pressure, allergic reactions, constipation, and opioid addiction.
[18][30]: 99 Taken orally, the naloxone has virtually no effect, due to the drug's extremely high first-pass metabolism and low bioavailability (2%).
Anecdotally, posters on drug-related online forums have stated that they were able to attain a high by injecting preparations of buprenorphine despite being combined with naloxone.
[35] Both buprenorphine and methadone are medications used for detoxification and opioid replacement therapy, and appear to have similar effectiveness based on limited data.
[36] Both are safe for pregnant women with opioid use disorder,[30]: 101 [32] although preliminary evidence suggests that methadone is more likely to cause neonatal abstinence syndrome.
Alternatively, up to a month's supply of buprenorphine has been able to be prescribed by clinicians in the US or Europe who have completed basic training (8–24 hours in the US) and received a waiver/licence allowing the prescription of the medicine.
[38] In 2021, seeking to address record levels of opioid overdose, the United States also removed the requirement for a special waiver for prescribing physicians.
[42][45] Common adverse drug reactions associated with the use of buprenorphine, similar to those of other opioids, include nausea and vomiting, drowsiness, dizziness, headache, memory loss, cognitive and neural inhibition, perspiration, itchiness, dry mouth, shrinking of the pupils of the eyes (miosis), orthostatic hypotension, male ejaculatory difficulty, decreased libido, and urinary retention.
[18] The usual reversal agents for opioids, such as naloxone, may be only partially effective, and additional efforts to support breathing may be required.
[32] In the setting of acute pain management, though, buprenorphine appears to cause the same rate of respiratory depression as other opioids such as morphine.
[93] Buprenorphine and norbuprenorphine may be quantified in blood or urine to monitor use or non-medical recreational use, confirm a diagnosis of poisoning, or assist in a medicolegal investigation.
A significant overlap of drug concentrations exists in body fluids within the possible spectrum of physiological reactions ranging from asymptomatic to comatose.
In the years before buprenorphine/naloxone was approved, Reckitt Benckiser had lobbied Congress to help craft the Drug Addiction Treatment Act of 2000, which gave authority to the Secretary of Health and Human Services to grant a waiver to physicians with certain training to prescribe and administer schedule III, IV, or V narcotic drugs for the treatment of addiction or detoxification.
This was eventually modified to allow approved physicians to treat up to 100 people with buprenorphine for opioid addiction in an outpatient setting.
[103] On 14 January 2021, the US Department of Health and Human Services announced that the waiver would no longer be required to prescribe buprenorphine to treat up to 30 people concurrently.
[105] In the European Union, Subutex and Suboxone, buprenorphine's high-dose sublingual tablet preparations, were approved for opioid use disorder treatment in September 2006.
[106] In the Netherlands, buprenorphine is a list II drug of the Opium Law, though special rules and guidelines apply to its prescription and dispensation.
In France, buprenorphine prescription by general practitioners and dispensed by pharmacies has been permitted since the mid-1990s as a response to HIV and overdose risk.
Deaths caused by heroin overdose were reduced by four-fifths between 1994 and 2002, and the incidence of AIDS among people who inject drugs in France fell from 25% in the mid-1990s to 6% in 2010.
[108] Some jails consider the more expensive form a positive tradeoff: a single monthly injection may be simpler and easier for the staff to manage than daily trips to the dispensary to have a nurse provide a pill and make sure that it has been swallowed.
[108] Buprenorphine is available under the brand names Cizdol, Brixadi (approved in the US by FDA for addiction treatment in 2023), Suboxone (with naloxone), Subutex (typically used for opioid use disorder), Zubsolv, Bunavail, Buvidal (approved in the UK, Europe and Australia for addiction treatment in 2018), Sublocade (approved in the US in 2018),[109][110][111] Probuphine, Temgesic (sublingual tablets for moderate to severe pain), Buprenex (solutions for injection often used for acute pain in primary-care settings), Norspan, and Butrans (transdermal preparations used for chronic pain).
[114] A buprenorphine implant (developmental code name SK-2110) is under development by Shenzhen ScienCare Pharmaceutical in China for the treatment of refractory major depressive disorder.
[117][118] Buprenorphine has been used in the treatment of the neonatal abstinence syndrome,[119] a condition in which newborns exposed to opioids during pregnancy demonstrate signs of withdrawal.
[120] In the United States, use currently is limited to infants enrolled in a clinical trial conducted under an FDA-approved investigational new drug (IND) application.