Prochirality
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step.
[2] If two identical substituents are attached to an sp3-hybridized atom, the descriptors pro-R and pro-S are used to distinguish between the two.
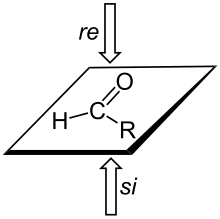
A trigonal planar sp2-hybridized atom can be converted to a chiral center when a substituent is added to the re or si (from Latin rectus 'right' and sinister 'left') face of the molecule.
Alexander Ogston[5] pointed out that when a symmetrical molecule is placed in an asymmetric environment, such as the surface of an enzyme, supposedly identically placed groups become distinguishable.
In this way he showed that earlier exclusion of non-chiral citrate as a possible intermediate in the tricarboxylate cycle was mistaken.