Descriptor (chemistry)
In chemical nomenclature, a descriptor is a notational prefix placed before the systematic substance name, which describes the configuration or the stereochemistry of the molecule.
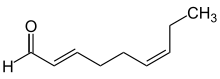
See: cis–trans isomerism The descriptors cis (Latin, on this side of)[2] and trans (Latin, over, beyond)[3] are used in various contexts for the description of chemical configurations:[4][5] In organic structural chemistry, the configuration of a double bond can be described with cis and trans, in case it has a simple substitution pattern with only two residues.
The position of two residues relative to one another at different points in a ring system or a larger molecule can also be described with cis and trans if the structure's configuration is rigid and does not allow simple inversion.
See: Arene substitution pattern The abbreviation o- (short for ortho, from Ancient Greek ὀρθός, meaning "upright, straight"),[8] m- (meta, μετά, (roughly) "between")[9] and p- (para, παρά, "adjoining, to the side")[10] describe the three possible positional isomers of two substituents on a benzene ring.
These are usually two independent single substituents, but in case of fused ring systems, ortho-fusing is also mentioned unless the substitution pattern is regarded in the name like in [2.2]paracyclophane.
The use of syn and anti to indicate the configuration of double bonds is nowadays obsolete, especially in case of aldoximes and hydrazones derived from aldehydes.
The terms fac (from Latin facies, 'external face')[16] and mer (from 'meridional')[17] can specify the arrangement of three identical ligands around the central atom in octahedral complexes.
[18][19] The prefix fac describes the situation when the three identical ligands occupy the three vertices of an octahedron triangular surface.
n, iso and neo are no longer used in the systematic nomenclature, but still frequently in trivial names and in laboratory jargon.
[25] In organic compounds, "cyclo" is frequently used as a name component, not separated by a hyphen and also considered in alphabetical sorting.
The terms sec and tert are considered obsolete and should only be used for unsubstituted sec-butoxy, sec-butyl[26][27] or tert-butyl groups.
[31] The term catena (Latin: "chain") is used in the inorganic nomenclature[32] to describe linear, chain-like polymers from identical polyatomic units.
Typographically, (R) and (S) are placed in uppercase and italic; the frequently preceding locants, the enclosing round brackets and the commas, on the other hand, as normal.
If the residue located on this carbon atom (usually an OH group) points to the left, the molecule originates from the L-series.
[41] The descriptors D- and L- are written as small capitals and separated by a hyphen from the rest of the name.