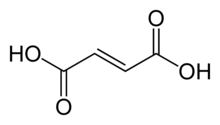
Fumaric acid
Fumaric acid is found in fumitory (Fumaria officinalis), bolete mushrooms (specifically Boletus fomentarius var.
Fumarate is an intermediate in the citric acid cycle used by cells to produce energy in the form of adenosine triphosphate (ATP) from food.
The European Commission Scientific Committee on Animal Nutrition, part of DG Health, found in 2014 that fumaric acid is "practically non-toxic" but high doses are probably nephrotoxic after long-term use.
In patients with relapsing-remitting multiple sclerosis, the ester dimethyl fumarate (BG-12, Biogen) significantly reduced relapse and disability progression in a phase 3 trial.
It activates the Nrf2 antioxidant response pathway, the primary cellular defense against the cytotoxic effects of oxidative stress.
[12] Fumaric acid is used in the manufacture of polyester resins and polyhydric alcohols and as a mordant for dyes.
[15] A traditional synthesis involves oxidation of furfural (from the processing of maize) using chlorate in the presence of a vanadium-based catalyst.
This weak acid forms a diester, it undergoes bromination across the double bond,[17] and it is a good dienophile.