Purkinje cell
Purkinje cells mainly release GABA (gamma-aminobutyric acid) neurotransmitter, which inhibits some neurons to reduce nerve impulse transmission.
[7] This has led to the notion that a "highly conserved one-to-one relationship renders Purkinje dendrites into a single computational compartment".
[11][12] PCP4 accelerates both the association and dissociation of calcium (Ca2+) with calmodulin (CaM) in the cytoplasm of Purkinje cells, and its absence impairs the physiology of these neurons.
[15] During early development Purkinje cells arise in the ventricular zone in the neural tube, the nervous system´s precursor in the embryo.
[18] This spatio-temporal distribution pattern suggests that neurogenins are involved in the specification of phenotypically heterogeneous Purkinje cell subsets, ultimately responsible for constructing the framework of the cerebellar topography.
P-type calcium channels were named after Purkinje cells, where they were initially encountered (Llinas et al. 1989), which are crucial in cerebellar function.
[29] Findings have suggested that Purkinje cell dendrites release endocannabinoids that can transiently downregulate both excitatory and inhibitory synapses.
[34] Numerical modeling of experimental data suggests that, in vivo, the Na+-K+ pump produces long quiescent punctuations (>> 1 s) to Purkinje neuron firing; these may have a computational role.
Some domestic animals can develop a condition where the Purkinje cells begin to atrophy shortly after birth, called cerebellar abiotrophy.
[43] A similar condition known as cerebellar hypoplasia occurs when Purkinje cells fail to develop in utero or die off before birth.
The genetic conditions ataxia telangiectasia and Niemann Pick disease type C, as well as cerebellar essential tremor, involve the progressive loss of Purkinje cells.
[44] Purkinje cells can also be damaged by the rabies virus as it migrates from the site of infection in the periphery to the central nervous system.





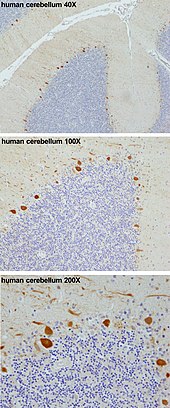
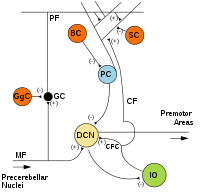
MF: Mossy fiber .
DCN: Deep cerebellar nuclei .
IO: Inferior olive .
CF: Climbing fiber .
GC: Granule cell .
PF: Parallel fiber .
PC: Purkinje cell.
GgC: Golgi cell .
SC: Stellate cell .
BC: Basket cell .