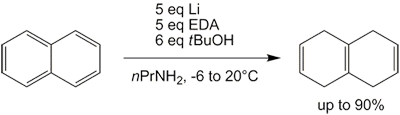Birch reduction
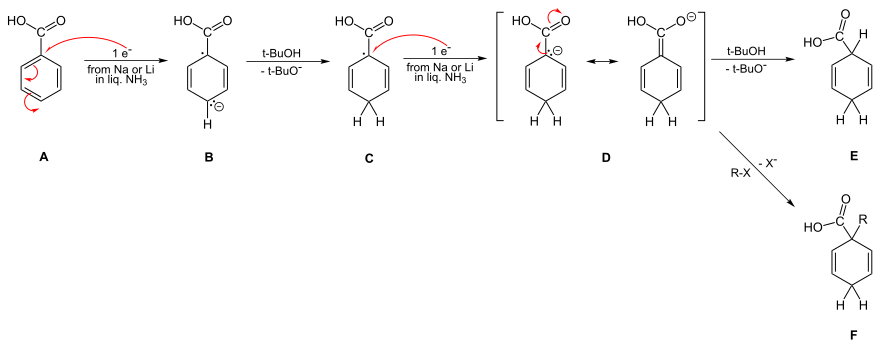
The solvated electrons add to the aromatic ring to give a radical anion, which then abstracts a proton from the alcohol.
Modifying the exchange integrals to account for varying interatomic distances, produces maximum electron density at the central atom 1,[7][8][9] a result confirmed by more modern RHF computations.
[7][11] Traditional Birch reduction requires cryogenic temperatures to liquify ammonia and pyrophoric alkali-metal electron donors.
The reduction can also be powered by an external potential or sacrificial anode (magnesium or aluminum), but then alkali metal salts are necessary to colocate the reactants via complexation.
[18] The Benkeser reduction is the hydrogenation of polycyclic aromatic hydrocarbons, especially naphthalenes using lithium or calcium metal in low molecular weight alkyl amines solvents.
Unlike traditional Birch reduction, the reaction can be conducted at temperatures higher than the boiling point of ammonia (−33 °C).
[25] Birch's original procedure used sodium and ethanol,[5][26][27] Alfred L. Wilds later discovered that lithium gives better yields.
[30][31][32] For electron-donating substituents, Birch initially proposed meta attack, corresponding to the location of greatest electron density in a neutral benzene ring, a position endorsed by Krapcho and Bothner-By.
[4][33] These conclusions were challenged by Zimmerman in 1961, who computed electron densities of the radical and diene anions, revealing that the ortho site which was most negative and thus most likely to protonate.