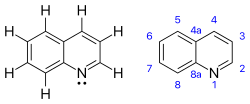
Quinoline
However, the German chemist August Hoffmann eventually recognized that the differences in behaviors was due to the presence of contaminants and that the two compounds were actually identical.
[10] The only report of quinoline as a natural product is from the Peruvian stick insect Oreophoetes peruana.
[11] Like other nitrogen heterocyclic compounds, such as pyridine derivatives, quinoline is often reported as an environmental contaminant associated with facilities processing oil shale or coal, and has also been found at legacy wood treatment sites.
Quinoline is readily degradable by certain microorganisms, such as Rhodococcus species Strain Q1, which was isolated from soil and paper mill sludge.
[12] Quinolines are present in small amounts in crude oil within the virgin diesel fraction.
Going clockwise from top these are: A number of other processes exist, which require specifically substituted anilines or related compounds: Quinolines are reduced to tetrahydroquinolines enantioselectively using several catalyst systems.
[8] The reduction of quinoline with sodium borohydride in the presence of acetic acid is known to produce Kairoline A.