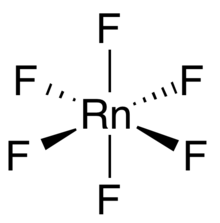
Radon hexafluoride
Radon hexafluoride is a binary chemical compound of radon and fluorine with the chemical formula RnF6.
[1][2][3] This is still a hypothetical compound that has not been synthesized so far.
The compound is calculated to be less stable than radon difluoride.
Radon hexafluoride is expected to have an octahedral molecular geometry, unlike the C3v of xenon hexafluoride.
[4][5] The Rn-F bonds in radon hexafluoride is predicted to be shorter and more stable compared to Xe-F bonds in xenon hexafluoride.