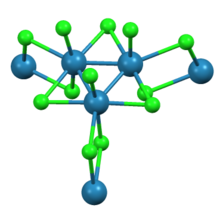
Trirhenium nonachloride
It is a dark red hygroscopic solid that is insoluble in ordinary solvents.
As shown by X-ray crystallography trirhenium nonachloride consists of Re3Cl12 subunits that share three chloride bridges with adjacent clusters.
[4] Trirhenium nonachloride is efficiently prepared by thermal decomposition of rhenium pentachloride or hexachlororhenic(IV) acid:[5] If the sample is vacuum sublimed at 500 °C, the resulting material is comparatively unreactive.
Other synthetic methods include treating rhenium with sulfuryl chloride.
[2] It is also obtained by heating Re2(O2CCH3)4Cl2 under HCl: Reaction of the tri- and pentachlorides gives rhenium tetrachloride: