Ammonium perrhenate
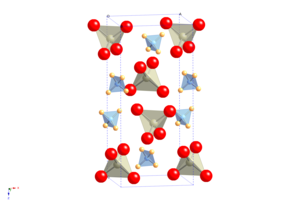
[3] The crystal structure of APR resembles that of scheelite, with atomic cation is replaced by ammonium.
[4] It undergoes a molecular orientational ordering transition on cooling without change of space group, but with a highly anisotropic change in the shape of the unit cell, resulting in the unusual property of having a positive temperature and pressure Re NQR coefficient.
[2] Ammonium perrhenate may be prepared from virtually all common sources of rhenium.
Alternatively an aqueous solution of Re2O7 can be treated with ammonia followed by crystallisation.
It slowly reacts with hydrochloric acid:[2] It is reduced to metallic Re upon heating under hydrogen:[1] Ammonium perrhenate decomposes to volatile Re2O7 starting at 250 °C.