Rhenium disulfide
The layers are held together by weak Van der Waals bonds, and can be easily peeled off from the bulk material.
[3] It can be synthesized from the reaction between rhenium and sulfur at 1000 °C, or the decomposition of rhenium(VII) sulfide at 1100 °C:[4] Nanostructured ReS2 can usually be achieved through mechanical exfoliation, chemical vapor deposition (CVD), and chemical and liquid exfoliations.
Larger crystals can be grown with the assistance of liquid carbonate flux at high pressure.
It is widely used in electronic and optoelectronic device, energy storage, photocatalytic and electrocatalytic reactions.
[6] These monolayers have shown layer-independent electrical, optical, and vibrational properties much different from other TMDs.
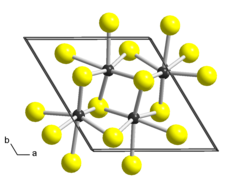
Different crystal structures were proposed for ReS2 based on single-crystal X-ray diffraction studies.
While all authors agree that the lattice is triclinic, the reported cell parameters and atomic arrangements slightly differ.
There are two layers in this unit cell, related by symmetry centers, and the chains of clusters run along the a axis.