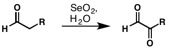
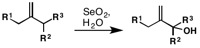
Riley oxidation
[4][5][6][7] The oxidation of carbonyl alpha methylene positions begins with attack by the enol tautomer at the electrophilic selenium center.
[8][9]: 4331 Allylic oxidation using selenium-dioxide proceeds via an ene reaction at the electrophilic selenium center.
The (E)- orientation about the double bond, a consequence of the envelope-like transition state, is observed in the penultimate ester formation, is retained during the hydrolysis step giving the (E)-allylic alcohol product.
In bridged ring systems, Bredt’s rule is followed and bridgehead positions are not oxidized: In their strychnine total synthesis, R.B.
Epimerization of the alpha hydrogen led to cis-glyoxal, which spontaneously underwent cyclization with the secondary amine to yield dehydrostryninone.