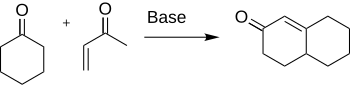
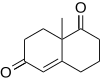
Robinson annulation
Formation of cyclohexenone and derivatives are important in chemistry for their application to the synthesis of many natural products and other interesting organic compounds such as antibiotics and steroids.
Initial approaches coupled the methyl vinyl ketone with a naphthol to give a naphtholoxide, but this procedure was not sufficient to form the desired cyclohexenone.
Subsequent aldol type ring closure leads to the keto alcohol, which is then followed by dehydration to produce the annulation product.
Alternatively, the regioselectivity is often controlled by using a β-diketone or β-ketoester as the enolate component, since deprotonation at the carbon flanked by the carbonyl groups is strongly favored.
In order to avoid a reaction between the original enolate and the cyclohexenone product, the initial Michael adduct is often isolated first and then cyclized to give the desired octalone in a separate step.
The trans compound is favored due to antiperiplanar effects of the final aldol condensation in kinetically controlled reactions.
As is the case with Robinson annulation, Michael addition usually happens first to tether the two reactants together, then aldol reaction proceeds intramolecularly to generate the ring system in the product.
The Dieckmann condensation is a similar ring closing intramolecular chemical reaction of diesters with base to give β-ketoesters.
[14] F. Dean Toste and co-workers[16] have used Robinson annulation in the total synthesis of (+)-fawcettimine, a tetracyclic Lycopodium alkaloid that has potential application to inhibiting the acetylcholine esterase.
Scientists at Merck discovered platensimycin, a novel antibiotic lead compound with potential medicinal applications as seen in the adjacent picture.
[17] Initial synthesis gave a racemic form of the compound using an intramolecular etherification reaction of the alcohol motifs and the double bond.
Yamamoto and coworkers report the use of an alternative intramolecular Robinson annulation to provide a straightforward enantioselective synthesis of tetracyclic core of platensimycin.