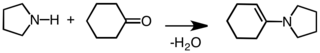Enamine
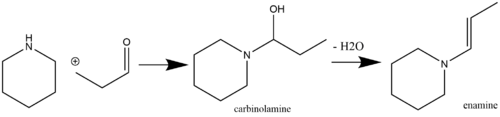
A common route for enamine production is via an acid-catalyzed nucleophilic reaction of ketone[7] or aldehyde[8] species containing an α-hydrogen with secondary amines.
[10] Primary amines are usually not used for enamine synthesis due to the preferential formation of the more thermodynamically stable imine species.
[11] Methyl ketone self-condensation is a side-reaction which can be avoided through the addition of TiCl4[12] into the reaction mixture (to act as a water scavenger).
The enamine starting material undergoes a nucleophilic addition to acyl halides forming the iminium salt intermediate which can hydrolyze in the presence of acid.
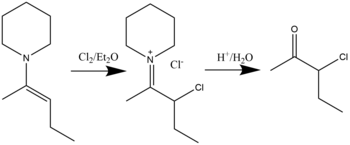
[18] β-halo immonium compounds can be synthesized through the halogenation reaction of enamines with halides in diethyl ether solvent.
The general reaction is shown below: Enamines can be efficiently cross-coupled with enol silanes through treatment with ceric ammonium nitrate.
These reactions were reported by the Narasaka group in 1975, providing a route to stable enamines as well as one instance of a 1,4-diketone (derived from a morpholine amine reagent).
[20] Later, these results were exploited by the MacMillan group with the development of an organocatalyst which used the Narasaka substrates to produce 1,4 dicarbonyls enantioselectively, with good yields.
[28][29] There are many ways to modulate enamine reactivity in addition to altering the steric/electronics at the nitrogen center including changing temperature, solvent, amounts of other reagents, and type of electrophile.