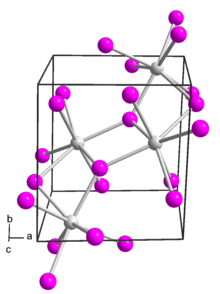
Samarium(II) iodide
In solid samarium(II) iodide, the metal centers are seven-coordinate with a face-capped octahedral geometry.
Although samarium(II) iodide is considered a powerful single-electron reducing agent, it does display remarkable chemoselectivity among functional groups.
For example, sulfones and sulfoxides can be reduced to the corresponding sulfide in the presence of a variety of carbonyl-containing functionalities (such as esters, ketones, amides, aldehydes, etc.).
[10] Tosyl groups can be removed from N-tosylamides almost instantaneously, using SmI2 in conjunction with distilled water and an amine base.
[12][13][14] The book Organic Synthesis Using Samarium Diiodide, published in 2009, gives a detailed overview of reactions mediated by SmI2.