Cyclic compound
Adding to their complexity and number, closing of atoms into rings may lock particular atoms with distinct substitution (by functional groups) such that stereochemistry and chirality of the compound results, including some manifestations that are unique to rings (e.g., configurational isomers).
Indeed, the development of this important chemical concept arose historically in reference to cyclic compounds.
[4] The vast majority of cyclic compounds are organic, and of these, a significant and conceptually important portion are composed of rings made only of carbon atoms (i.e., they are carbocycles).
[4] Indeed, the development of this important chemical concept arose, historically, in reference to cyclic compounds.
For instance, cyclohexanes—six membered carbocycles with no double bonds, to which various substituents might be attached, see image—display an equilibrium between two conformations, the chair and the boat, as shown in the image.
[4] Which of the possible chair conformations predominate in cyclohexanes bearing one or more substituents depends on the substituents, and where they are located on the ring; generally, "bulky" substituents—those groups with large volumes, or groups that are otherwise repulsive in their interactions[citation needed]—prefer to occupy an equatorial location.
Hence, if forced into the higher energy boat form, these methyl groups are in steric contact, repel one another, and drive the equilibrium toward the chair conformation.
In organic chemistry, the term aromaticity is used to describe a cyclic (ring-shaped), planar (flat) molecule that exhibits unusual stability as compared to other geometric or connective arrangements of the same set of atoms.
In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.
Hofmann used the term for a class of benzene compounds, many of which do have odors (aromas), unlike pure saturated hydrocarbons.
In terms of the electronic nature of the molecule, aromaticity describes a conjugated system often made of alternating single and double bonds in a ring.
[citation needed] Because of the unique shapes, reactivities, properties, and bioactivities that they engender, cyclic compounds are the largest majority of all molecules involved in the biochemistry, structure, and function of living organisms, and in the man-made molecules (e.g., drugs, herbicides, etc.)
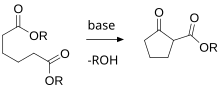
Examples include: A variety of further synthetic procedures are particularly useful in opening carbocyclic and other rings, generally which contain a double bound or other functional group "handle" to facilitate chemistry; these are termed ring-opening reactions.
Ring expansions and contractions can involve the insertion of a functional group such as the case with Baeyer–Villiger oxidation of cyclic ketones, rearrangements of cyclic carbocycles as seen in intramolecular Diels-Alder reactions, or collapse or rearrangement of bicyclic compounds as several examples.