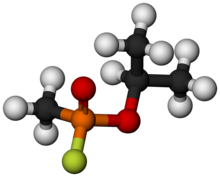
Sarin
[4] People who absorb a non-lethal dose and do not receive immediate medical treatment may suffer permanent neurological damage.
Production and stockpiling of sarin was outlawed as of April 1997 by the Chemical Weapons Convention of 1993, and it is classified as a Schedule 1 substance.
[7] Initial symptoms following exposure to sarin are a runny nose, tightness in the chest, and constriction of the pupils (miotic action).
Common mnemonics for the symptomatology of organophosphate poisoning, including sarin, are the "killer Bs" of bronchorrhea and bronchospasm because they are the leading cause of death,[8] and SLUDGE – salivation, lacrimation, urination, defecation, gastrointestinal distress, and emesis (vomiting).
Death may follow in one to ten minutes after direct inhalation, but may also occur after a delay ranging from hours to several weeks, in cases where exposure is limited but no antidote is applied.
[7] Sarin has a high volatility (ease with which a liquid can turn into vapour) relative to similar nerve agents, making inhalation very easy, and may even absorb through the skin.
Biperiden, a synthetic acetylcholine antagonist, has been suggested as an alternative to atropine due to its better blood–brain barrier penetration and higher efficacy.
[10] Sarin is a potent inhibitor of acetylcholinesterase,[11] an enzyme that degrades the neurotransmitter acetylcholine after it is released into the synaptic cleft.
In vertebrates, acetylcholine is the neurotransmitter used at the neuromuscular junction, where signals are transmitted between neurons from the peripheral nervous system to muscle fibres.
Controlled studies in healthy men have shown that a nontoxic 0.43 mg oral dose administered in several portions over a 3-day interval caused average maximum depressions of 22 and 30%, respectively, in plasma and erythrocyte acetylcholinesterase levels.
A single acute 0.5 mg dose caused mild symptoms of intoxication and an average reduction of 38% in both measures of acetylcholinesterase activity.
The serum level of unbound isopropyl methylphosphonic acid (IMPA), a sarin hydrolysis product, ranged from 2–135 μg/L in survivors of a terrorist attack during the first four hours post-exposure.
Sarin or its metabolites may be determined in blood or urine by gas or liquid chromatography, while acetylcholinesterase activity is usually measured by enzymatic methods.
One clear advantage of this process is that the period, post-exposure, for determination of sarin exposure is much longer, possibly five to eight weeks according to at least one study.
[28] The scheme below shows a generic example that employs the Di-Di method as the final esterification step; in reality, the selection of reagents and reaction conditions dictate both product structure and yield.
[28] As both reactions leave considerable acid in the product, sarin produced in bulk by these methods has a short half life without further processing, and would be corrosive to containers and damaging to weapons systems.
[41] Sarin's shelf life can be extended by increasing the purity of the precursor and intermediates and incorporating stabilizers such as tributylamine.
[42] In mid-1939, the formula for the agent was passed to the chemical warfare section of the German Army Weapons Office, which ordered that it be brought into mass production for wartime use.
[43] Though sarin, tabun, and soman were incorporated into artillery shells, Germany did not use nerve agents against Allied targets.