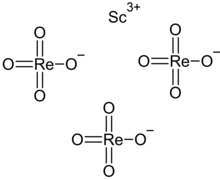
Scandium perrhenate
Scandium perrhenate is an inorganic compound, with the chemical formula Sc(ReO4)3.
Its thermal stability is lower than that of the corresponding compounds of the yttrium and lanthanum perrhenates.
[2][3] Scandium perrhenate can be obtained by reacting perrhenic acid with scandium oxide.
[1] From the solution, the trihydrate of scandium perrhenate can be precipitated, which loses water at 50 °C to obtain Sc(ReO4)3·H2O, and obtains the anhydrous form at 140 °C.
[4] Scandium perrhenate trihydrate is a crystal in the triclinic crystal system, with space group P1, a=7.333, b=7.985, c=20.825 Å; α=93.35, β=92.20, γ=97.42°.