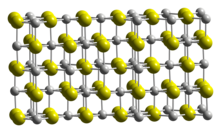
Scandium(III) sulfide
Whereas NaCl has all the octahedral interstices in the anion lattice occupied by cations, Sc2S3 has one third of them vacant.
The vacancies are ordered, but in a very complicated pattern, leading to a large, orthorhombic unit cell belonging to the space group Fddd.
[1] Metal sulfides are usually prepared by heating mixtures of the two elements, but in the case of scandium, this method yields scandium monosulfide, ScS.
Sc2S3 can be prepared by heating scandium(III) oxide under flowing hydrogen sulfide in a graphite crucible to 1550 °C or above for 2–3 hours.
[1] Scandium(III) sulfide can be prepared by reacting scandium(III) chloride with dry hydrogen sulfide at elevated temperature:[2] Above 1100 °C, Sc2S3 loses sulfur, forming nonstoichiometric compounds such as Sc1.37S2.