Sea ice
Polar packs undergo a significant yearly cycling in surface extent, a natural process upon which depends the Arctic ecology, including the ocean's ecosystems.
Sea ice can be classified according to whether or not it is attached (or frozen) to the shoreline (or between shoals or to grounded icebergs).
[6][7] Another classification used by scientists to describe sea ice is based on age, that is, on its development stages.
The ice cover may also undergo a state of tension, resulting in divergence and fissure opening.
If two floes drift sideways past each other while remaining in contact, this will create a state of shear.
[6][7] A new ridge is a recent feature – it is sharp-crested, with its side sloping at an angle exceeding 40 degrees.
[6][7] Leads and polynyas are areas of open water that occur within sea ice expanses even though air temperatures are below freezing.
They are also used for navigation purposes – even when refrozen, the ice in leads is thinner, allowing icebreakers access to an easier sail path and submarines to surface more easily.
[11] Convection of the surface layer involves the top 100–150 m (330–490 ft), down to the pycnocline of increased density.
At a certain point such a disc shape becomes unstable and the growing isolated crystals take on a hexagonal, stellar form, with long fragile arms stretching out over the surface.
With any kind of turbulence in the water, these fragments break up further into random-shaped small crystals which form a suspension of increasing density in the surface water, an ice type called frazil or grease ice.
In rough water, fresh sea ice is formed by the cooling of the ocean as heat is lost into the atmosphere.
He applied this theory in the field in the Kara Sea, which led to the discovery of Vize Island.
In the Arctic, the area of ocean covered by sea ice increases over winter from a minimum in September to a maximum in March or sometimes February, before melting over the summer.
The presence of melt ponds is affected by the permeability of the sea ice (i.e. whether meltwater can drain) and the topography of the sea ice surface (i.e. the presence of natural basins for the melt ponds to form in).
They also have lower albedo since they are on thinner ice, which blocks less of the solar radiation from reaching the dark ocean below.
[20] Both brine and air volumes influence sea-ice density values, which are typically around 840–910 kg/m3 for first-year ice.
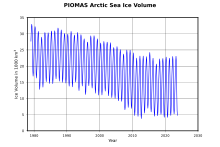
Sea-ice density is a significant source of errors in sea-ice thickness retrieval using radar and laser satellite altimetry, resulting in uncertainties of 0.3–0.4 m.[21] Changes in sea ice conditions are best demonstrated by the rate of melting over time.
A composite record of Arctic ice demonstrates that the floes' retreat began around 1900, experiencing more rapid melting beginning within the past 50 years.
Antarctic sea ice extent gradually increased in the period of satellite observations, which began in 1979, until a rapid decline in southern hemisphere spring of 2016.
The bright, shiny surface (albedo) of the ice also serves a role in maintaining cooler polar temperatures by reflecting much of the sunlight that hits it back into space.
[5] In order to gain a better understanding about the variability, numerical sea ice models are used to perform sensitivity studies.
There are many sea ice model computer codes available for doing this, including the CICE numerical suite.
Many global climate models (GCMs) have sea ice implemented in their numerical simulation scheme in order to capture the ice–albedo feedback correctly.
When sea water freezes, the ice is riddled with brine-filled channels which sustain sympagic organisms such as bacteria, algae, copepods and annelids, which in turn provide food for animals such as krill and specialised fish like the bald notothen, fed upon in turn by larger animals such as emperor penguins and minke whales.
[25] A decline of seasonal sea ice puts the survival of Arctic species such as ringed seals and polar bears at risk.
Scientists notably suspect the existence of "icebergs" of solid diamond and corresponding seas of liquid carbon on the ice giants, Neptune and Uranus.
This is due to extreme pressure and heat at the core, that would turn carbon into a supercritical fluid.