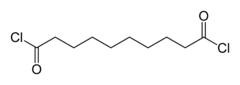
Sebacoyl chloride
A colorless oily liquid with a pungent odor, it is soluble in hydrocarbons and ethers.
It is less susceptible to hydrolysis though than shorter chain aliphatic acyl chlorides.
[1] Sebacoyl chloride can be prepared by reacting sebacic acid with an excess of thionyl chloride.
Residual thionyl chloride can be removed by distillation.
[2] Sebacoyl chloride can be polymerized with hexamethylenediamine yielding nylon-6,10.