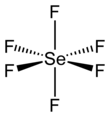
Selenium hexafluoride
The relative reactivity of the hexafluorides of S, Se, and Te follows the order TeF6 > SeF6 > SF6, the latter being completely inert toward hydrolysis until high temperatures.
[3] The gas can be passed through 10% NaOH or KOH without change, but reacts with gaseous ammonia at 200 °C.
[8] Although selenium hexafluoride is quite inert and slow to hydrolyze, it is toxic even at low concentrations,[9] especially by longer exposure.
In the U.S., OSHA and ACGIH standards for selenium hexafluoride exposure is an upper limit of 0.05 ppm in air averaged over an eight-hour work shift.
Additionally, selenium hexafluoride is designated as IDLH chemical with a maximum allowed exposure limit of 2 ppm.