Selenium tetrachloride
[3] When the reacting selenium is heated, the product sublimes from the reaction flask.
As such, one would predict four bonds but five electron groups giving rise to a seesaw geometry.
This formulation would predict a pyramidal geometry for the SeCl3+ cation with a Cl-Se-Cl bond angle of approximately 109°.
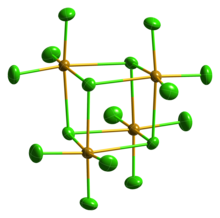
However, this molecule is an excellent example of a situation where maximal bonding cannot be achieved with the simplest molecular formula.
The formation of the tetramer (SeCl4)4,[5] with delocalized sigma bonding of the bridging chloride is clearly preferred over a "hypervalent" small molecule.