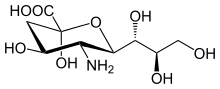
Sialic acid
[1] The term "sialic acid" (from Greek σίαλον (síalon) 'saliva') was first introduced by Swedish biochemist Gunnar Blix in 1952.
[3][4][5][6] Sialic acids are commonly part of glycoproteins, glycolipids or gangliosides, where they decorate the end of sugar chains at the surface of cells or soluble proteins.
[8] The hydroxyl substituents may vary considerably; acetyl, lactyl, methyl, sulfate, and phosphate groups have been found.
Normally they can be found as components of oligosaccharide chains of mucins, glycoproteins and glycolipids occupying terminal, nonreducing positions of complex carbohydrates on both external and internal membrane areas where they are very exposed and develop important functions.
This enzyme uses for example a mannose derivative as a substrate, inserting three carbons from pyruvate into the resulting sialic acid structure.
Since water is a polar molecule with partial positive charges on both hydrogen atoms, it is attracted to cell surfaces and membranes.
[18] Sialic acid can "hide" mannose antigens on the surface of host cells or bacteria from mannose-binding lectin.
Administration of estrogen to castrated mice leads to a dose-dependent reduction of the sialic acid content of the vagina.
Sialic acids provide a good target for these viruses since they are highly conserved and are abundant in large numbers in virtually all cells.
Unsurprisingly, sialic acids also play an important role in several human viral infections.
Widely used anti-influenza drugs (oseltamivir and zanamivir) are sialic acid analogs that interfere with release of newly generated viruses from infected cells by inhibiting the viral enzyme neuraminidase.
For example, evidence indicates that free sialic acids can behave as a signal to some specific bacteria, like Pneumococcus.
Free sialic acid possibly can help the bacterium to recognize that it has reached a vertebrate environment suitable for its colonization.
The synthesis starts in the cytosol, where N-acetylmannosamine 6 phosphate and phosphoenolpyruvate give rise to sialic acid.
Some severe diseases can depend on the presence or absence of some enzymes related to the sialic acid metabolism.
Sialidosis and Sialic acid deficiency with mutations in the NANS gene (see below) would be examples of this type of disorder.
[22] Rat pups supplemented with sialic acid showed improved learning and memory as adults.
[25] A therapeutic trial with a short-term supplementation of sialic acid given orally has failed to show a significant beneficial effect on biochemical parameters [26] Salla disease is an extremely rare illness which is considered the mildest form of the free sialic acid accumulation disorders[27] though its childhood form is considered an aggressive variant and people who suffer from it have mental retardation.
[33] Many pathogenic bacteria incorporate sialic acid into cell surface features like their lipopolysaccharide or capsule polysaccharides, which helps them to evade the innate immune response of the host.