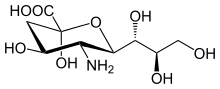
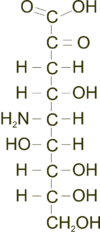
Neuraminic acid
[1] Neuraminic acid does not occur naturally, but many of its derivatives are found widely distributed in animal tissues and in bacteria, especially in glycoproteins and gangliosides.
The hydroxyl substituents may vary considerably: acetyl, lactyl, methyl, sulfate and phosphate groups have been found.
The name "neuraminic acid" was introduced by German scientist E. Klenk in 1941, in reference to the brain lipids from which it was derived as a cleavage product.
[2] The IUPAC symbol used for neuraminic acid is Neu, and the residue is typically found with additional chemical modifications in biological systems.
Among their many biological functions, these structures are substrates for neuraminidase enzymes which cleave neuraminic acid residues.