Red blood cell
[2] Erythrocytes take up oxygen in the lungs, or in fish the gills, and release it into tissues while squeezing through the body's capillaries.
[12] Vertebrate red blood cells consist mainly of hemoglobin, a complex metalloprotein containing heme groups whose iron atoms temporarily bind to oxygen molecules (O2) in the lungs or gills and release them throughout the body.
The blood plasma alone is straw-colored, but the red blood cells change color depending on the state of the hemoglobin: when combined with oxygen the resulting oxyhemoglobin is scarlet, and when oxygen has been released the resulting deoxyhemoglobin is of a dark red burgundy color.
[14] Pulse oximetry takes advantage of the hemoglobin color change to directly measure the arterial blood oxygen saturation using colorimetric techniques.
Flushed, confused patients with a saturation reading of 100% on pulse oximetry are sometimes found to be suffering from carbon monoxide poisoning.
Members of this order have clearly evolved a mode of red blood cell development substantially different from the mammalian norm.
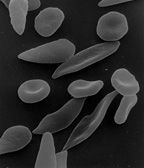
[10][17] Overall, mammalian red blood cells are remarkably flexible and deformable so as to squeeze through tiny capillaries, as well as to maximize their apposing surface by assuming a cigar shape, where they efficiently release their oxygen load.
The red blood cells without nuclei, called reticulocytes, subsequently lose all other cellular organelles such as their mitochondria, Golgi apparatus and endoplasmic reticulum.
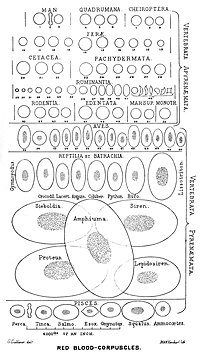
In comparison, the red blood cells of other vertebrates have nuclei; the only known exceptions are salamanders of the family Plethodontidae, where five different clades has evolved various degrees of enucleated red blood cells (most evolved in some species of the genus Batrachoseps), and fish of the genus Maurolicus.
[25][26][27] The elimination of the nucleus in vertebrate red blood cells has been offered as an explanation for the subsequent accumulation of non-coding DNA in the genome.
[17] The argument runs as follows: Efficient gas transport requires red blood cells to pass through very narrow capillaries, and this constrains their size.
In the absence of nuclear elimination, the accumulation of repeat sequences is constrained by the volume occupied by the nucleus, which increases with genome size.
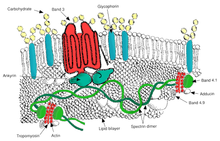
Additionally, there are also "scramblase" proteins that move phospholipids in both directions at the same time, down their concentration gradients in an energy-independent manner.
The maintenance of an asymmetric phospholipid distribution in the bilayer (such as an exclusive localization of PS and PIs in the inner monolayer) is critical for the cell integrity and function due to several reasons: The presence of specialized structures named "lipid rafts" in the red blood cell membrane have been described by recent studies.
These are structures enriched in cholesterol and sphingolipids associated with specific membrane proteins, namely flotillins, STOMatins (band 7), G-proteins, and β-adrenergic receptors.
Recall that respiration, as illustrated schematically here with a unit of carbohydrate, produces about as many molecules of carbon dioxide, CO2, as it consumes of oxygen, O2.
First, because, besides hemoglobin, they contain a large number of copies of the enzyme carbonic anhydrase on the inside of their cell membrane.
[41] The H+ ions released by this rapid reaction within RBC, while still in the capillary, act to reduce the oxygen binding affinity of hemoglobin, the Bohr effect.
In summary, carbon dioxide produced by cellular respiration diffuses very rapidly to areas of lower concentration, specifically into nearby capillaries.
Red blood cells can also produce hydrogen sulfide, a signalling gas that acts to relax vessel walls.
It is believed that the cardioprotective effects of garlic are due to red blood cells converting its sulfur compounds into hydrogen sulfide.
[55][56] Furthermore, the pentose phosphate pathway plays an important role in red blood cells; see glucose-6-phosphate dehydrogenase deficiency for more information.
[59] The inability to carry out protein synthesis means that no virus can evolve to target mammalian red blood cells.
[63] The aging red blood cell undergoes changes in its plasma membrane, making it susceptible to selective recognition by macrophages and subsequent phagocytosis in the mononuclear phagocyte system (spleen, liver and lymph nodes), thus removing old and defective cells and continually purging the blood.
[64] This process normally occurs at the same rate of production by erythropoiesis, balancing the total circulating red blood cell count.
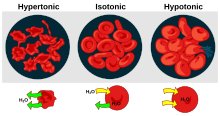
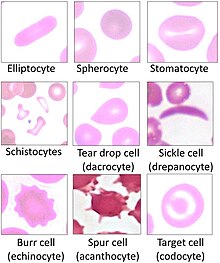
Eryptosis can be elicited by osmotic shock, oxidative stress, and energy depletion, as well as by a wide variety of endogenous mediators and xenobiotics.
In the 1740s, Vincenzo Menghini in Bologna was able to demonstrate the presence of iron by passing magnets over the powder or ash remaining from heated red blood cells.
A year later Alfred von Decastello and Adriano Sturli, two colleagues of Landsteiner, identified a fourth blood group—AB.
In 1959, by use of X-ray crystallography, Max Perutz was able to unravel the structure of hemoglobin, the red blood cell protein that carries oxygen.
[72] The oldest intact red blood cells ever discovered were found in Ötzi the Iceman, a natural mummy of a man who died around 3255 BCE.