Signal transduction
The nature of such stimuli can vary widely, ranging from extracellular cues, such as the presence of EGF, to intracellular events, such as the DNA damage resulting from replicative telomere attrition.
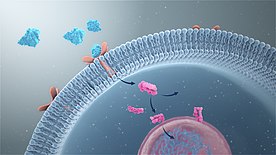
In addition, some molecules such as steroid hormones are lipid-soluble and thus cross the plasma membrane to reach cytoplasmic or nuclear receptors.
Such signaling is mainly orchestrated in focal adhesions, regions where the integrin-bound actin cytoskeleton detects changes and transmits them downstream through YAP1.
[17] Cellular and systemic control of osmotic pressure (the difference in osmolarity between the cytosol and the extracellular medium) is critical for homeostasis.
[21] Additionally, animal cells contain a conserved mechanism to prevent high temperatures from causing cellular damage, the heat-shock response.
[21] In mammals, light controls the sense of sight and the circadian clock by activating light-sensitive proteins in photoreceptor cells in the eye's retina.
[23] In the case of the circadian clock, a different photopigment, melanopsin, is responsible for detecting light in intrinsically photosensitive retinal ganglion cells.
[27] Once the GPCR recognizes a ligand, the conformation of the receptor changes to activate the G protein, causing Gα to bind a molecule of GTP and dissociate from the other two G-protein subunits.
Subsequent to this, the receptors' kinase domains are activated, initiating phosphorylation signaling cascades of downstream cytoplasmic molecules that facilitate various cellular processes such as cell differentiation and metabolism.
Ligand binding to the extracellular domain of integrins changes the protein's conformation, clustering it at the cell membrane to initiate signal transduction.
[37] As shown in the adjacent picture, cooperative integrin-RTK signaling determines the timing of cellular survival, apoptosis, proliferation, and differentiation.
[42][43][44] These adapters activate other intracellular molecules such as IRAK1, IRAK4, TBK1, and IKKi that amplify the signal, eventually leading to the induction or suppression of genes that cause certain responses.
All hormones that act by regulation of gene expression have two consequences in their mechanism of action; their effects are produced after a characteristically long period of time and their effects persist for another long period of time, even after their concentration has been reduced to zero, due to a relatively slow turnover of most enzymes and proteins that would either deactivate or terminate ligand binding onto the receptor.
The ligand-binding domain is additionally responsible for dimerization of nucleic receptors prior to binding and providing structures for transactivation used for communication with the translational apparatus.
The HSPs are necessary to activate the receptor by assisting the protein to fold in a way such that the signal sequence enabling its passage into the nucleus is accessible.
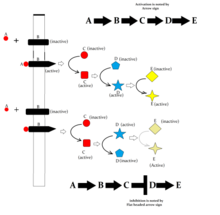
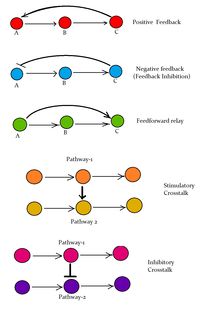
Receptor activity can be enhanced by phosphorylation of serine residues at their N-terminal as a result of another signal transduction pathway, a process called crosstalk.
[45][46] First messengers are the signaling molecules (hormones, neurotransmitters, and paracrine/autocrine agents) that reach the cell from the extracellular fluid and bind to their specific receptors.
In essence, second messengers serve as chemical relays from the plasma membrane to the cytoplasm, thus carrying out intracellular signal transduction.
Examples include diacylglycerol and ceramide, the former required for the activation of protein kinase C. Nitric oxide (NO) acts as a second messenger because it is a free radical that can diffuse through the plasma membrane and affect nearby cells.
In addition to nitric oxide, other electronically activated species are also signal-transducing agents in a process called redox signaling.
[48] Gene activations[49] and metabolism alterations[50] are examples of cellular responses to extracellular stimulation that require signal transduction.
Hence, an initial stimulus can trigger the expression of a large number of genes, leading to physiological events like the increased uptake of glucose from the blood stream[50] and the migration of neutrophils to sites of infection.
Such requirements for extracellular stimulation are necessary for controlling cell behavior in unicellular and multicellular organisms; signal transduction pathways are perceived to be so central to biological processes that a large number of diseases are attributed to their dysregulation.
Following are some major signaling pathways, demonstrating how ligands binding to their receptors can affect second messengers and eventually result in altered cellular responses.
He noted that guanosine triphosphate disassociated glucagon from this receptor and stimulated the G-protein, which strongly influenced the cell's metabolism.
[64][65] Widespread use of the term has been traced to a 1980 review article by Rodbell:[60][66] Research papers focusing on signal transduction first appeared in large numbers in the late 1980s and early 1990s.
Thus, within a relatively short time a plausible model was developed for the molecular basis of immunological specificity, and for mediation of biological function through the Fc domain.
Crystallization of an IgG molecule soon followed [69] ) confirming the inferences based on sequencing, and providing an understanding of immunological specificity at the highest level of resolution.
The first hints of this were obtained by Becker et al [71] who demonstrated that the extent to which human basophils—for which bivalent Immunoglobulin E (IgE) functions as a surface receptor – degranulate, depends on the concentration of anti IgE antibodies to which they are exposed, and results in a redistribution of surface molecules, which is absent when monovalent ligand is used.
The first of these was a simple model proposed by Bell [74] which resolved an apparent paradox: clustering forms stable networks; i.e. binding is essentially irreversible, whereas the affinities of antibodies secreted by B cells increase as the immune response progresses.