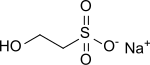
Sodium 2-hydroxyethyl sulfonate
[1] Sodium 2-hydroxyethyl sulfonate is formed by the reaction of ethylene oxide with sodium hydrogen sulfite in aqueous solution: To avoid contamination and suppress the formation of by-products (which are difficult to remove) the reaction must be performed under careful control of mass ratios and process conditions.
[2] Excess sulfite (SO32−) or bisulfite (HSO3−) lead to an unpleasant odor of the downstream product, higher levels of ethylene glycol or glycol ethers (formed by the hydrolysis and ethoxylation of ethylene oxide) give hygroscopic and greasy surfactants.
The reaction has to take place under the exclusion of oxygen and under precise control of the stoichiometry of the reactants, the temperature, the pH and the throughput.
These are readily foaming and particularly mild, making them suitable for cleaning sensitive skin and are therefore mainly used in baby soaps and shampoos.
[6] The addition of 2-hydroxyethyl sulfonate to electroplating baths allows higher current densities and lower concentrations than the much more expensive methane sulphonic acid with improved appearance.